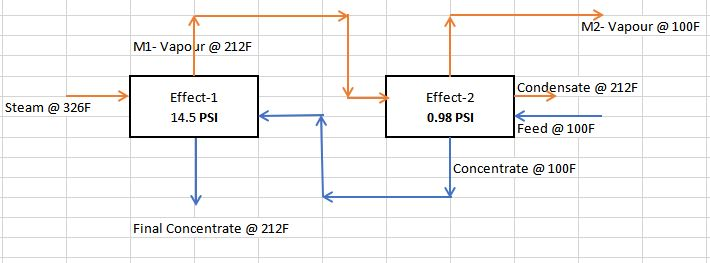
1. A feed containing 2 wt% dissolved organic solids in water is fed to a double-effect evaporator with reverse feed. The feed enters at 100°F and is concentrated to 25% solids. The boiling- point rise can be considered negligible. Each evaporator has a 1000 ft² surface area, and the heat transfer coefficients are U1=500, and U2=700 btu/h.ft.F. The feed enters evaporator number 2 and steam at 100 psia is fed to number 1. The pressure in the vapour space of evaporator number 2 is 0.98 psia. Assume that the heat capacity of all liquid solutions is that of liquid water. Calculate the feed rate Lj of a solution containing 25 % solids. (Hint: Assume a feed rate of, say F=1000 lbm/h and a value of T). Calculate the area and T1. Then calculate the actual feed rate by multiplying 1000 by 1000/calculated area.)
Answer :


2.
Answer :
As per the given data:
Solids in feed= 2%
Solids in concentrate= 25%
HTA1 = HTA2 = 1000 ft^2
U1 = 500 Btu/ h ft^2 F & U2 = 700 Btu/ h ft^2 F
Overall material balance: Feed= Distillate + concentrate ----> Eq-1
Component balance: Feed * 0.02 = Distillate * 0 + concentrate * 0.25
Feed = 12.5 * concentrate ---> Eq-2
Boiling point rise = negligible, so solution & solvent vapor temperature will be same.
Assumed that the 1st effect is operating under atmospheric pressure (Boiling point - 212F).
As per the data:
Latent heat @ 212F = 300 Btu/lb
Latent heat @ 100F = 320 Btu/lb
As per material balance:
Vapor flowrate * latent heat = Overall HT coefficient * HTA * DT
1st effect: M-1= (500 * 1000 * (326-212)) / 300 = 190,000 lb/hr
2nd effect: M-2= (700*1000 * (212-100)) / 320 = 245,000 lb/hr
Distillate = M-1 + M-2 = 190,000+245,000 = 435,000 lb/hr
Substituting the above in Eq-1
Feed = 435,000 + concentrate
Substitute Eq-2 in the above
12.5 * concentrate = 435,000 + concentrate
Concentrate, L1 = 435,000/11.5 = 37826 lb /hr
Feed, F = 435,000 + 37826 = 472,826 lb/hr
1st effect operating pressure is not given, That may be the reason we are not getting the given answer. But procedure is right.
3.
Question :
A single-effect evaporator is concentrating a feed of 9072 g/h of a 10 wt% solution of NaOH in water to a product of 50% solids. The pressure of the saturated steam used is 42 kPa (gage) and the pressure in the vapor space of the evaporator is 20 kPa (abs). The overall heat-transfer coefficient is 1988 W/m2*K. a) calculate the steam use, steam economy and area if the feed temperature at 288.8 k (15.6 c) b) predict the new product composition if the feed rate is reduced to 4536 kg/h
Answer :




![Av = 0.027m2-7 J767 (VTT Sts z U, A. (TS-T) 3 x 2260 1988 x36wy 0.027 (383-333) S 2 4275 g/h] = S[HP (VMI Economy ( S 7257.6](https://media.cheggcdn.com/coop/92a/92acff9b-560f-47cf-915a-3fb6d2c61657/1620673855205_20210511_003301.jpg)
Question :
A single effect evaporator is concentrating a feed of 9072 kg/h of a 10 wt% solution of NaOH in water to a product of 50% solids. The pressure of the saturated steam used is 42 kPa(gage) and the pressure in the vapor space of the evaporator is 20 kpa (abs). The overall heat transfer coefficient is 1988W/mk. Calculate the steam used, the steam economy in kg/vaporized/kg steam, and the area for the following feed conditions : a. Feed temperature of 288.8k(15.6c) b. Feed temperature of 322.1k (48.9 c)
Answer :

![(Col feed = 4017 CkJ/kg) Energy balance givers (ms) a) [mseed Cpl T-TF) + (m:22] -CULA (T-TP) (ms) (2510) - [69072/3600) (4:0](https://media.cheggcdn.com/coop/686/686f44ca-aa23-431c-b6ab-8f6eab511dab/1605444674153_Screenshot_20201115-181945_Gallery.jpg)
Comments
Post a Comment